What is Nattokinase?




Efficacy of Natto and Nattokinase
Natto and Nattokinase
A traditional Japanese food “Natto” is a food made from soybeans fermented by Bacillus natto, and has been eaten since about 1,200 years ago. Enzymes that Bacillus natto produce by fermentation include protease and Dr. Oshima at Hokkaido Imperial University reported its purification and characteristics. Since then, it was investigated as a serine protease, and, in 1980s, the enzyme that degrades fibrin proteins (a cause of thrombi) was named as “Nattokinase”. Nattokinase is found in the sticky part of Natto.
Function of Nattokinase
Functions of Nattokinase include directly degrading a fibrin (the main component of thrombi), activating pro-urokinase (precursor for urokinase that is an thrombolytic enzyme in the body) and increasing the amount of tissue plasminogen activator (t-PA) that produces a thrombolytic enzyme, plasmin. In addition, recent research has revealed that Nattokinase has a function of degrading plasminogen activator inhibitor, PAI-1 and reducing the euglobulin lysis time, and therefore, it has a function of improving the thrombolytic activity. Furthermore, the reduction of blood pressure has also been confirmed.
Degradation of Thrombus by Nattokinase
The Role and Danger of Thrombus
A thrombus is a blood clot formed in blood mainly consisting of a protein called a fibrin, which is necessary for restoring the damaged blood vessel and stopping bleeding. Once bleeding stops and the damaged blood vessel is restored, thrombi are degraded. This phenomenon is called the fibrinolytic action. However, when there is an imbalance between the production of thrombus (coagulation) and its degradation (fibrinolysis), or the condition that thrombus are difficult to be degraded due to aging or stress, blood cots accumulate in blood vessels. These blood clots cause thrombosis such as cardiac infarction and cerebral infarction when they block the blood vessel of the heart and brain, respectively.
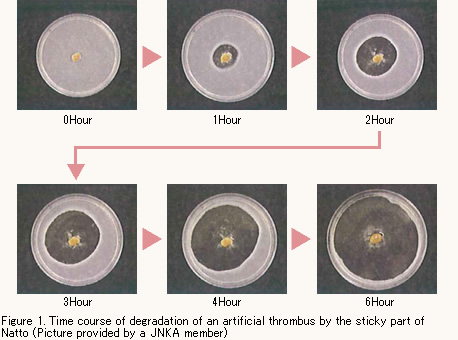
Degradation of Artificial Thrombus by Nattokinase
Figure 1 shows the time course of change in artificial thrombus (white part) on which Natto was placed. As you can see, the thrombus around Natto is degraded as time goes by. This degradation phenomenon (action) takes place because of Nattokinase in the sticky part of Natto.
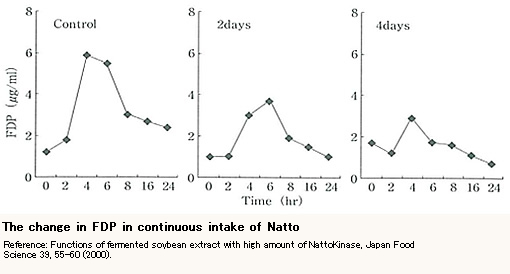
Intake of Natto and Fibrin Degradation Product (FDP)
Figure 2 below shows the change in fibrin degradation product (FDP) of 5 healthy volunteers who ate Natto, measured by latex agglutination. The graph named “Control” represents the result after eating Natto for the first time, “2 days” represents the result after eating Natto for the second time on the second day, and “4 days” represents the result after eating Natto for the third time on the fourth day. You can see that FDP value dramatically increased in 4 hours after eating Natto every time and decreased in 6 hours and 8 hours. Moreover, comparing those 3 charts, the FDP peak numbers went down as the volunteers ate Natto more times. This means that fibrins got degraded by continuously eating Natto.
TOPICS – Intestinal absorption of Nattokinase in animal experiments
It is confirmed that Nattokinase got absorbed by bowel and the absorbed Nattokinase degraded plasma fibrinogen.
<Reference>
Fujita M , Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, et al.
Transport of nattokinase across the rat intestinal tract.
Biol. Pharm. Bull. 1995;18(9):1194-6.
Intraduodenal administration of nattokinase (NK) at a dose of 80 mg/kg, resulted in the degradation of fibrinogen in plasma suggesting transport of NK across the intestinal tract in normal rats. The action of NK on the cleavage of fibrinogen in the plasma from blood samples drawn at intervals after intraduodenal administration of the enzyme was investigated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting analysis with an anti-fibrinogen γchain antibody. The 270 kDa fragment carrying antigenic sites for the binding of the anti-fibrinogen γchain antibody appeared within 0.5 h and was then degraded gradually to a 105 kDa fragment via a 200 kDa fragment. This suggests that fibrinogen was degraded to a 105 kDa fragment via several intermediates (270 and 200 kDa). In parallel with the degradation process, plasma recalcification times were remarkably prolonged. NK was also detected in the plasma from blood samples drawn 3 and 5 h after administration of the enzyme by SDS-PAGE and Western blotting analysis with an anti-NK antibody. The results indicate that NK is absorbed from the rat intestinal tract and that NK cleaves fibrinogen in plasma after inraduodenal administration of the enzyme.
Lysis inhibitor and Nattokinase
Depression of thrombolytic function can be a risk factor for thrombosis. It is decided by the balance of t-PA (a lysis promoter) and PAI-1 (a lysis inhibitor) that how readily thrombus gets lysed.
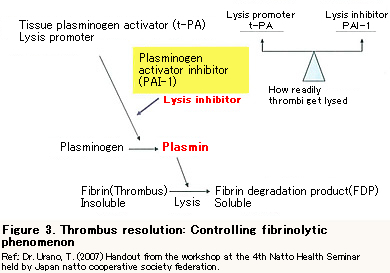
PAI-1 will increase due to many factors including obesity, high cholesterol, hypertriglyceridemia, insulin resistance (high blood sugar), stress and inflammation, where it is difficult for thrombus to get resolved. However, PAI-1 can be degraded (or inactivated) by Nattokinase.
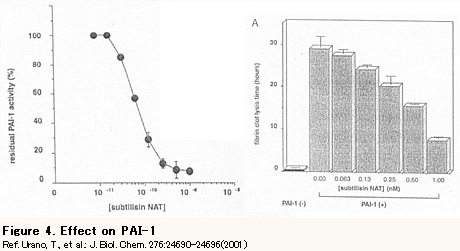
- Change of PAI-1 in a day (Thrombosis is more likely to happen at dawn)
Cerebral infarction and cardiac infarction happen more often in the early morning and it is considered that this is related to circadian rhythm of PAI-1. Figure 5 shows the circadian change of the PAI-1 level in the blood. It is high in the morning and it decreases as time goes; thus thrombus is more likely to be generated in the morning.
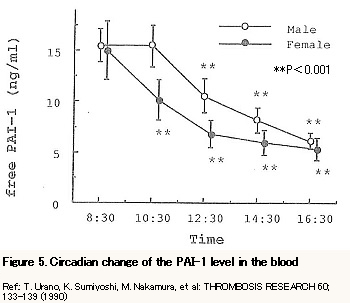
Shortening euglobulin clot lysis time by Nattokinase
Figure 6 represents that there is a significant difference in the result from the animal experiment (using rats) of comparison of ECLT (euglobulin clot lysis time) where experimental group was fed Nattokinase for three weeks. This shows the experimental group has got an advantage in the lytic state and therefore the blood viscosity was lowered.
Nottokinase for lifestyle-related disease prevention
Natto Foods vs. Nattokinase Supplements
The level of Nattokinase activity in commercially available natto foods varies and the activity level will decreases due to the way the Natto food is cooked or prepared. However, ingredients in dietary supplements are more stable. Therefore, supplements often offer a more stable level of Nattokinase activity.
It is often discussed that the best time to take Nattokinase is after dinner or before sleep. You may not like to eat Natto foods before sleep but it is easy to do so with supplements.
Natto foods have very distinctive smell and flavour so that you need consider where and when to eat them. However, you can take supplements wherever you like.
Natto foods have a high nutritional value but also a high amount of calorie and purine, which you may be concerned with. In contrast, calories in supplements are usually lower so that you do not need worry about the calorie intake.
About intake of Nattokinase
The recommended amount of Nattokinase is more than 2,000FU/ day.
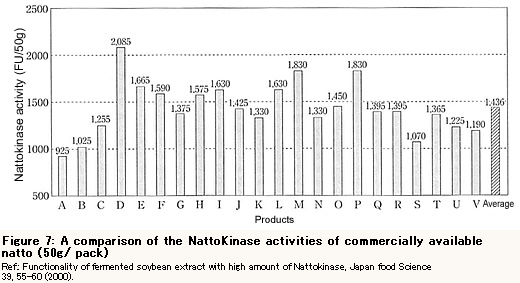
The Nattokinase activity level in natto commercially available varies from 1,400 FU/ pack (50g) to 2,000FU/ pack as Figure 7 shows.
The enzymatic activity of Nattokinase is indicated by FU which stands for Fibrin Degradation Unit.
Fibrin degradation method (using FU) is used to indicate a Nattokinase activity level as it is reproducible and accurate to quantify. (Figure 8)
It is considered best to take Nattokinase after dinner or before sleep since thrombus is more likely to be produced around the mid night to the early morning.
It is recommended to take Nattokinase on a regular basis for those who are over 40 years old, stressed-out, have relatively high blood pressure, and have high blood viscosity due to hyperlipidemia or diabetes.
*1. It is possible if incorrupt Nattokinase is used, however the apparent titer becomes low due to the low affinity compared to fibrin.
*2. “IU” can be used only when measuring plasmin activity.
*3.H-D-Val-Leu-Lys-pNA
*4. “IU” can be used only when measuring urokinase activity.
*5.pyro-Glu-Gly-Arg-pNA
*6. “U” could be used when converting lysis area to known fibrin-degrading enzyme activity.
Means of differentiating true Nattokinase from the counterfeit
Trends of counterfeit Nattokinase
Today, there are many counterfeit enzymes claiming to be Nattokinase available in the market, where it has been worried that those counterfeit products may affect the consumer’s health. If those counterfeit products are a protease, they can degrade fibrin in a test tube or on a petri dish to a greater or lesser extent. However, this does not mean that the same reactions could take place in vivo. Moreover, the safety is under question. Unfortunately, the assay method of Nattokinase cannot determine if an enzyme is Nattokinase. Therefore, the use of a means of differentiating Nattokinase from other proteases is necessary.
Simple differentiating method of Nattokinase (“IBOX method”)
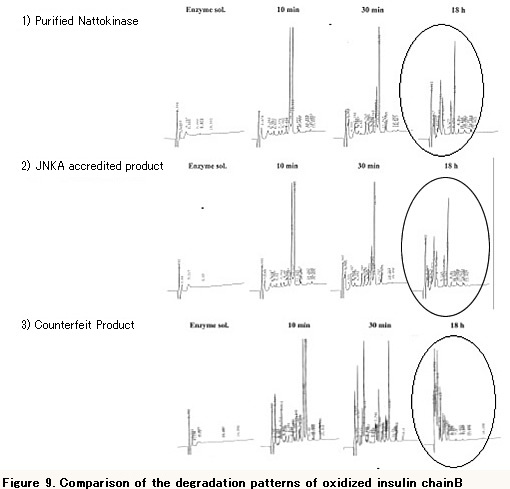
In the simple differentiating method of Nattokinase (called "IBox method"), an oxidized insulin chain B consisting of 30 amino acids is degraded by a sample enzyme and the degradation pattern is confirmed by HPLC. This method allows you to determin if the sample enzyme is Nattokinase because a different enzyme shows a different degradation pattern. Figure 9 shows the degradation patterns of oxidized insulin B-chain of 1) purified Nattokinase, 2) a JNKA accredited product and 3) a counterfeit product. Comparing the graphs, you can see that the degradation pattern of the counterfeit product is different from that of incorrupt Nattokinase; therefore you can tell that the counterfeit product does not contain Nattokinase. For further details, please contact Japan NattoKinase Association Secretariat.